Group A Streptococcus (GAS), also known as Streptococcus pyogens, is a common Gram-positive pathogen, which is the main pathogen causing acute tonsillitis, pharyngitis and scarlet fever in children. Some strains can cause serious complications such as rheumatic fever/rheumatic valvular heart disease and acute glomerulonephritis. GAS is one of the pathogens that can cause the most kinds of human diseases so far, and it is also one of the most common respiratory pathogens. It is easily confused with pharyngitis caused by mycoplasma pneumoniae and respiratory viruses in clinical practice. Accurate diagnosis of the causative agent is an important condition for correct treatment of the disease.
Bioer has developed a novel Real-Time PCR kit for the detection and identification of Group A Streptococcus. In addition, the kit also adds human internal control, monitoring the whole process of specimen collection, transportation, nucleic acid extraction, PCR amplification, to ensure the effectiveness of the whole process.
Fast detection speed: Fast detection speed, results can be obtained in about 1 hour.
Simple operation: One-step PCR is used to avoid aerosol contamination.
High sensitivity: Tested with three different batches of reagents, the detection sensitivity can reach 500 copies/mL.
Strong anti-interference ability: Potential interfering substances such as blood (10%), mucin (0.2mg/mL), hydroxymethyluracil (0.05mg/mL), sodium chloride (0.09%), betamethasone (0.05mg/mL), dexamethasone (0.1mg/L), flunisolide (0.1mg/mL), triamcinolone (105ng/mL), budesonide (3nmol/L), mometasone (0.2mg/mL), fluticasone (0.5ng/mL), histamine hydrochloride (1mg/mL), zanamivir (142ng/mL), ribavirin (3.6μg/mL), oseltamivir (0.02mg/mL), and paramivir (10μg/mL) have no effect on the detection results.
High precision: The CV values of the high and low concentration precision reference materials are both ≤5%.
Group A Streptococcus Nucleic Acid Detection Kit (Fluorescence PCR) is used forqualitative detection of Group A Streptococcus nucleic acid from human oropharyngeal swabs.
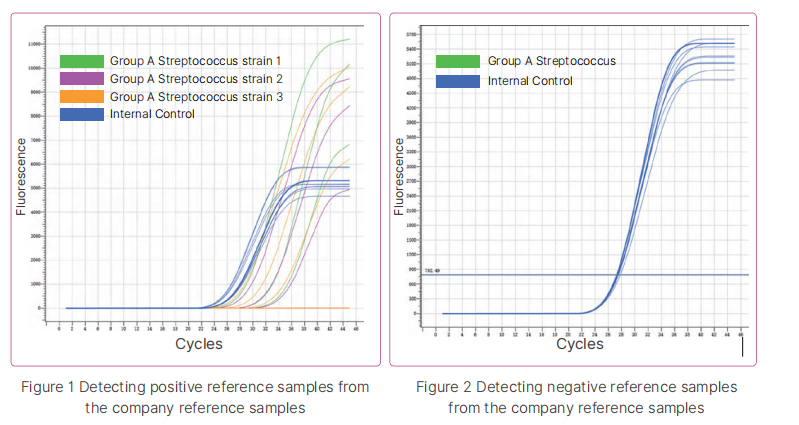
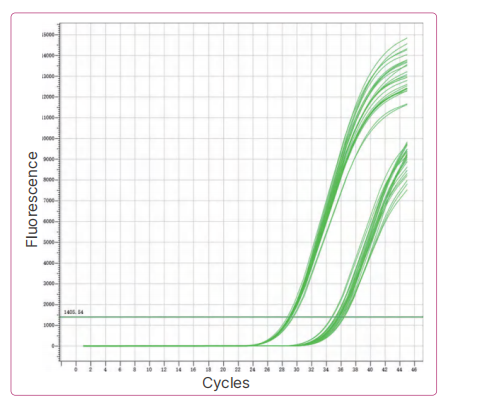
1. Accuracy: Bioer Technology positive reference samples P1-P9 and negative reference samples N1-N10 were extracted by using Bioer extraction reagent BSC86S1E as required, and then tested.
1. The kit should be stored at -20C+°C away from light.
2. The kit can be stored for up to 12 months if all components are kept in the manner above. Do not use after the stated expiry date.
3. The kit can be transported in foam box sealed with ice bags or dry ice under -8°C for 7 days.
4. After opening, the kit can be stored at 2°C-8°C for 7 days.
5. Avoid repeated freeze-thawing, and the number of freeze-thawing should not exceed 5 times.
| Cat# | Product Name | Packing size |
| BSJ38M1 | Group A Streptococcus Nucleic Acid Test Kit (Fluorescence PCR) | 48T |
| BSJ38L1 | Group A Streptococcus Nucleic Acid Test Kit (Fluorescence PCR) | 96T |

