Vibrio cholerae is a gram-negative bacterium that causes cholera, a severe diarrheal disease characterized by sudden onset of profuse, watery diarrhea, which can lead to dehydration and death if untreated. The primary mode of transmission is through contaminated water and food, making the disease particularly prevalent in areas with inadequate sanitation.
This Vibrio Cholerae Nucleic Acid Detection Kit (Fluorescence PCR) provides a highly sensitive and specific method for detecting the presence of V. cholerae in clinical samples. Unlike traditional culture methods, which can be time-consuming and less sensitive, fluorescence PCR allows for rapid, qualitative detection, offering results in a much shorter time frame. The fluorescent PCR approach not only improves accuracy by minimizing false positives and negatives but also enables the detection of low levels of bacterial nucleic acid, making it a superior tool for early diagnosis and effective outbreak management.
High Throughput and Broad Range Detection: The kit offers high detection throughput, capable of simultaneously testing up to 94 samples in a single 96-well experiment.
Rapid Detection: The entire detection process can be completed within 50 minutes.
Contamination-Free Operation: Fully enclosed tube operation eliminates the risk of contamination.
Sample Monitoring: A non-competitive human internal control is included to evaluate sample quality, identify PCR inhibitors, and prevent false-negative results.
1. Linear relationship
Dilute the positive reference sample from the company in a 10-fold gradient to 103 copies/mL and then perform the detection.
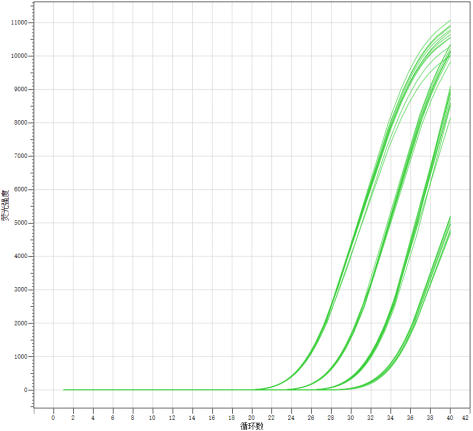
The results indicate that there is a good linear relationship when detecting the company's reference samples with this reagent kit.
2. Reproducibility
Dilute the positive reference sample from the company to 105 copies/mL and 500 copies/mL, and then perform the detection 20 times for each dilution. Calculate the coefficient of variation (CV) of the Ct values, with the requirement that the CV of the Ct values does not exceed 5%.
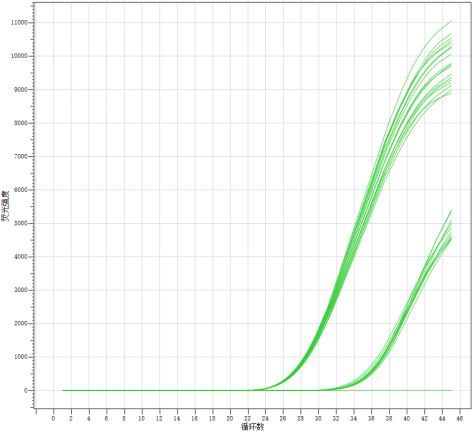
The results indicate that the precision of the 20 repeated detections of the company's reference samples at 105 copies/mL and 500 copies/mL concentrations is good, with the coefficient of variation (CV) of the Ct values being less than 5%, demonstrating that the reagent kit has good reproducibility.
1. The kit should be stored at -25°C ~ -15°C away from light, and avoid repeated freeze-thaw.
2. The kit can be stored for 5 days at 2-8 °C after opening.
3. The kit can be stored for up to 12 months if all components are kept in the manner above. Do not use after the stated expiry date.
4. The kit can be transported in foam box sealed with ice bags or dry ice under 8 ℃.
| Cat# | Product Name | Packing size |
| BSJ39M1 | Vibrio Cholerae Nucleic Acid Detection Kit (Fluorescence PCR) | 48T |
| BSJ39L1 | Vibrio Cholerae Nucleic Acid Detection Kit (Fluorescence PCR) | 96T |

