The Adenovirus and Type 3, 4, 7 Nucleic Acid Detection Kit (Fluorescent PCR) is designed for the rapid, specific, and qualitative detection of adenovirus DNA, including types 3, 4, and 7. Using real-time fluorescent PCR technology, the kit ensures high sensitivity and precision in identifying these viral strains, making it suitable for clinical diagnostics and public health monitoring. The assay features a streamlined protocol with minimal hands-on time, enabling reliable results for respiratory infections caused by adenovirus. It also includes internal controls to ensure accuracy and eliminate false-negative results.
This product selects the adenovirus (FAM), adenovirus type 3 (FAM), adenovirustype 4 (HEX) and adenovirus type 7 (ROX) to designs five sets of primers andfluorescent probes. The five sets of primers and probes can specifically bind to thetarget sequences. When the PCR amplification reaction is performed, thefluorescent signal(s) can be detected by a full-automatic fluorescent PCR detectorto realize real-time online monitoring of the PCR reaction. In order to control theentire extraction and detection process, human gene was act as a non-competitiveinternal control during the extraction and detection process.
Adenovirus is one of the main viruses that cause respiratory tract infection. This kit can judge whether there is adenovirus infection and whether it is infected with 3/4/7 types in one reaction, providing information for clinical diagnosis; at the same time, human-derived the internal reference monitors the entire process of specimen collection, transportation, nucleic acid extraction and PCR amplification; it takes only 60 minutes to complete the experiment.
High sensitivity: 500 copies/mL.
Strong specificity: With a variety of common respiratory infection pathogens such as: Bacillus pertussis, Moraxella catarrhalis, Aspergillus flavus, oral streptococcus, parainfluenza type 3, metapneumovirus A2, EV71, CB1, CA24, EV70 , HRVA30, HRV52, Measles virus, Boca virus, Influenza A H1N1 virus, Influenza B virus, Respiratory syncytial virus type A, Parainfluenza virus type 1, Rhinovirus type A2, Staphylococcus aureus, Haemophilus influenzae.
High accuracy:Detect adenovirus and type 3/4/7 at the same time. Test results of the reference product: the positive coincidence rate is 100%, and the negative coincidence rate is 100%.
The kit is used for the generic detection of adenovirus nucleic acid andtype-specific identification of adenovirus nucleic acid, cannot be used as the basisfor the diagnosis or exclusion of cases alone.
For professional use only.
For in vitro diagnostic use only.
Accuracy: The positive reference products P1-P11 and the negative reference products N1-N10 of the reference products of Hangzhou Bioer Technology Co., Ltd. were extracted using the Bioer extraction reagent BSC86S1E according to the instructions.
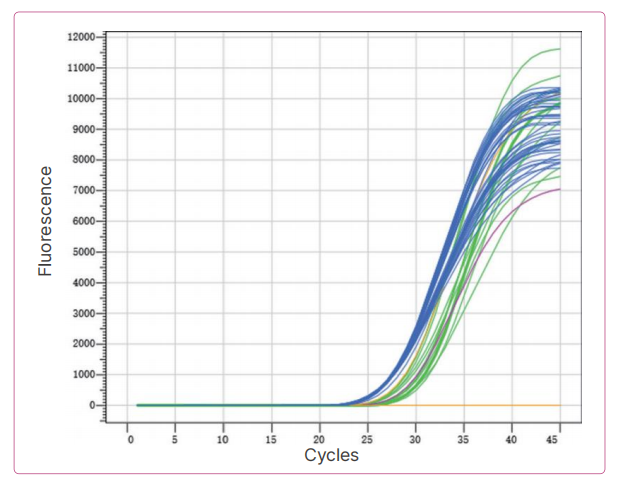
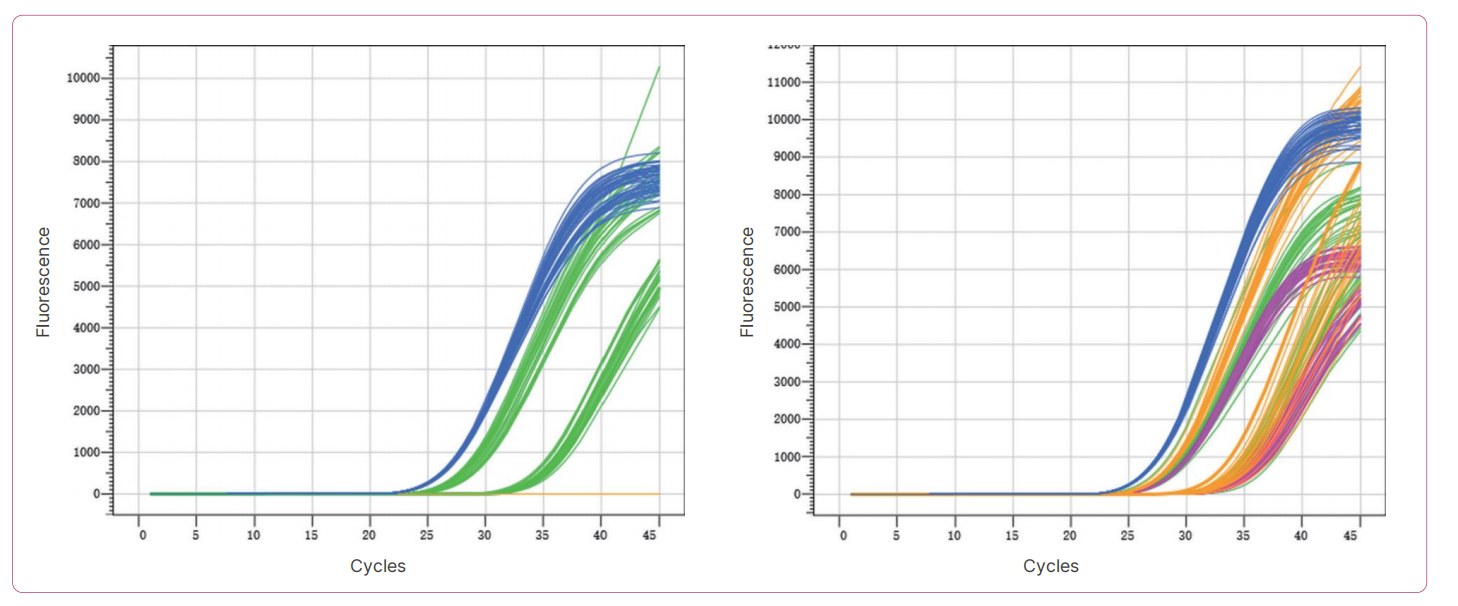
Result: The results showed that the precision variation coefficients of the three batches of reagents were all less than 5%, and the reagent precision was good.
1. The kit should be stored at -20C+°C away from light.
2. The kit can be stored for up to 12 months if all components are kept in the manner above. Do not use after the stated expiry date.
3. The kit can be transported in foam box sealed with ice bags or dry ice under -8°C for 7 days.
4. After opening, the kit can be stored at 2°C-8°C for 7 days.
5. Avoid repeated freeze-thawing, and the number of freeze-thawing should not exceed 5 times.
| Cat# | Product Name | Packing size |
| BSJ06S1 | Adenovirus and Type 3, 4, 7 Nucleic Acid Detection Kit (fluorescent PCR) | 24T |
| BSJ06M1 | Adenovirus and Type 3, 4, 7 Nucleic Acid Detection Kit (fluorescent PCR) | 48T |

