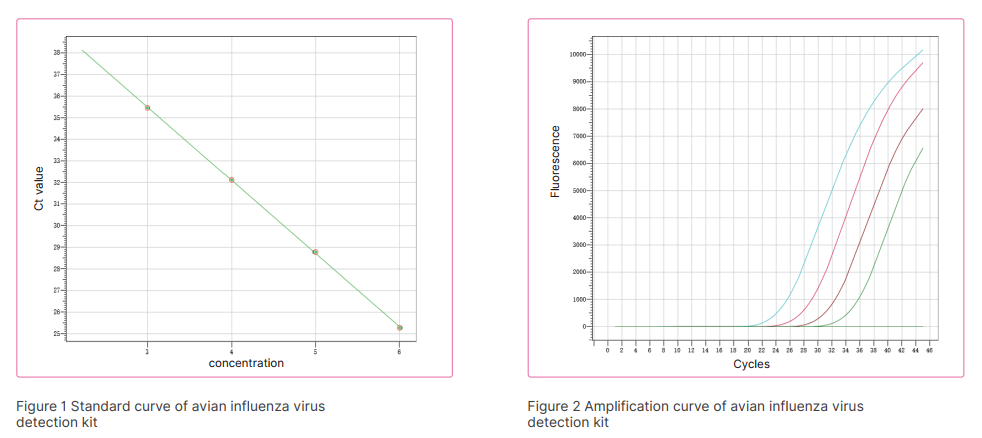
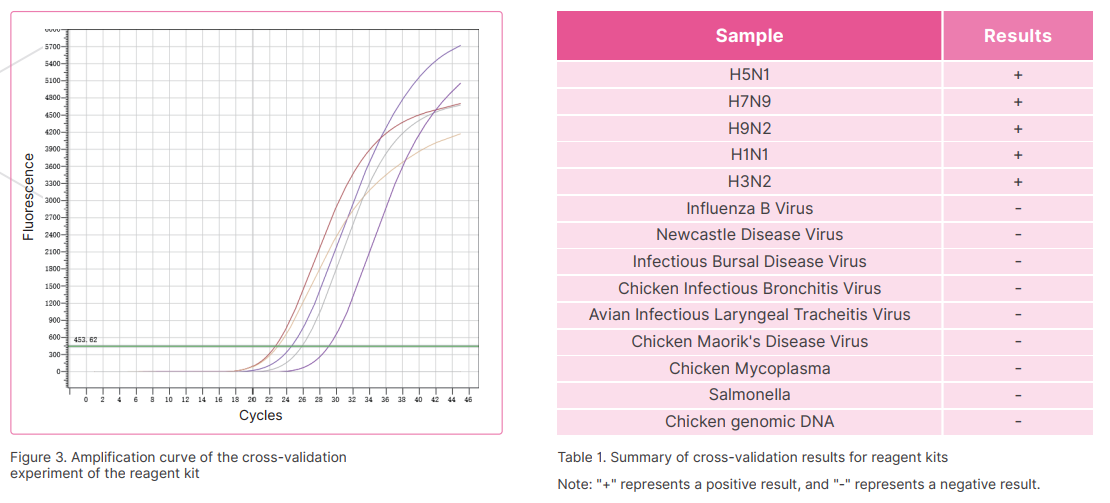
Avian influenza (AI) viruses, commonly known as bird flu, are members of the family orthmyxoviridae, Type A which is subdivided into categories (strains) depending on the outer proteins H (Haemagglutinin) and N (Neuraminidase). There are many types of avian influenza, which are all caused by various strains of type A influenza virus (e.g., H5N1, H7N3, H9N2). Avian influenza A(H5N1) illness is caused by the avian influenza A(H5N1) virus. The disease spreads by direct contact or through contaminated faeces and bodily fluids. New AI virus strains are created frequently which means that there is a constant risk that one of the new strains may spread easily among people. Avian Influenza continues to be a threat to the worldwide poultry industry. An effective control strategy for avian influenza in domesticated poultry is therefore an essential element in the protection of both bird and human populations. A laboratory test is required to diagnose Avian influenza (AI) viruses, Real-Time PCR technology has the advantages of high specificity and sensitivity, so it is recommended for laboratory detection. Based on its mature molecular diagnosis platform and technical reserves, Bioer Technology has developed related products of avian influenza virus nucleic acid detection reagents.
Quick Entrance
- CN | Japanese