Quick Entrance
- CN | Japanese

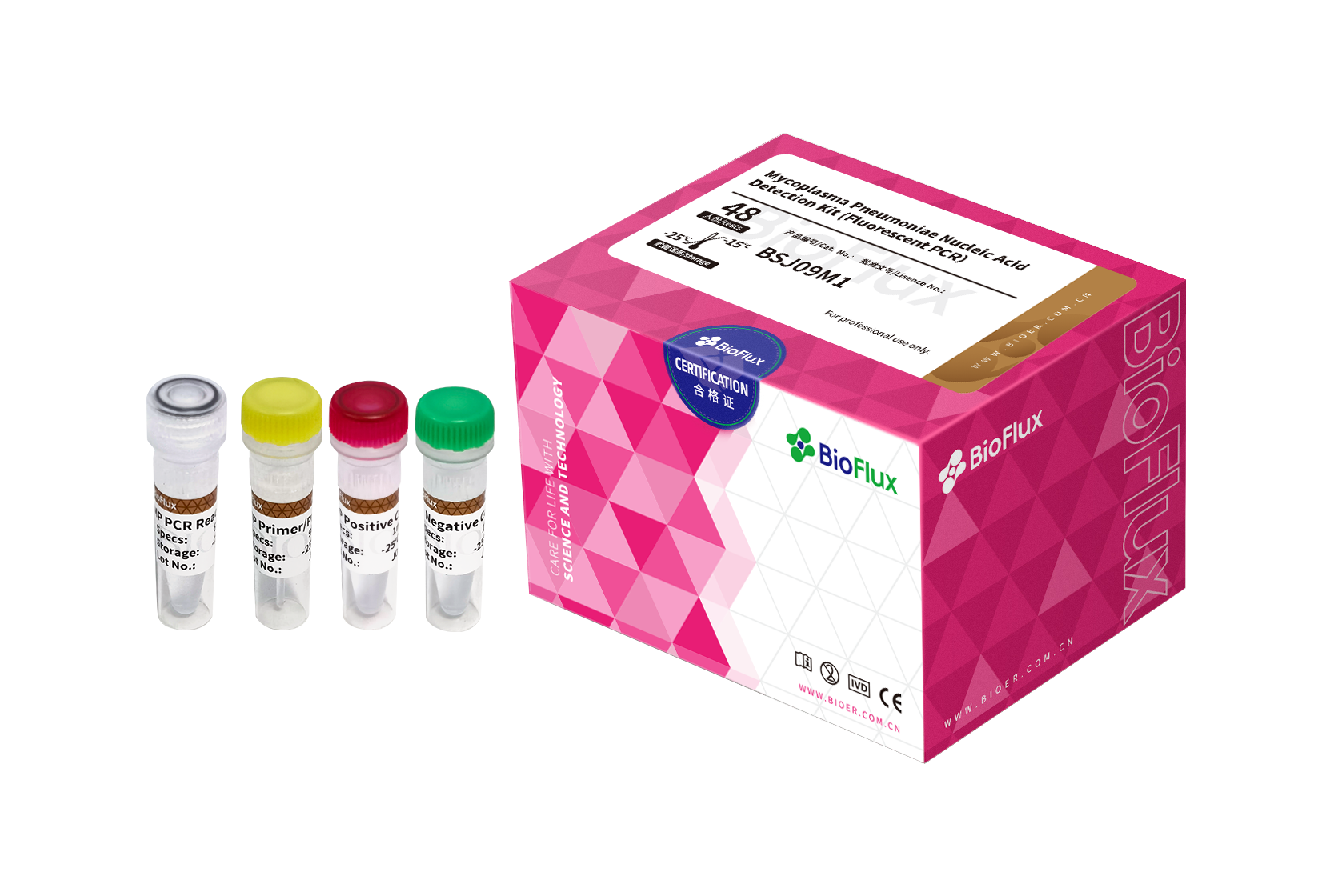
| Cat.No | Product Name | Package |
| BSJ09S1 | Mycoplasma Pneumoniae Nucleic Acid Detection Kit (Fluorescent PCR) | 24T |
| BSJ09M1 | Mycoplasma Pneumoniae Nucleic Acid Detection Kit (Fluorescent PCR) | 48T |
Case 1. Enterprise positive references P1-P10 and negative references N1-N10 were extracted with Bioer Technology's nucleic acid purification reagent and then tested by this product, the results are shown blow.
references were 100%, indicating that this kit has excellent performance.
Case 2. Nucleic acids were extracted using Bioer nucleic acid purification reagent after Mycoplasma pneumoniae samples were diluted by a gradient of 10 times. And the experiment was performed by this kit to test their amplification curves and standard curves.
Conclusion: The results showed that the correlation coefficients of Mycoplasma pneumoniae samples were above 0.995 and the linear relationship was good.
Case 3. The precision reference J1-J2 of this kit were redissolved according to the test. Nucleic acids were extracted using Bioer nucleic acid purification reagent and 3 batches of J1-J2 were tested by this kit. The whole tests were repeated 10 times to test the precision.
Conclusion: The results showed that the precision variation coefficients of the three batches of reagents
were all less than 5%, indicating that the reagents had good precision.
Case 4. The extracted nucleic acid of Mycoplasma Pneumoniae clinical samples were tested with this reagent. At the same time, the competitive reagent in the market were compared to verify the coincidence rate.