Quick Entrance
- CN | Japanese

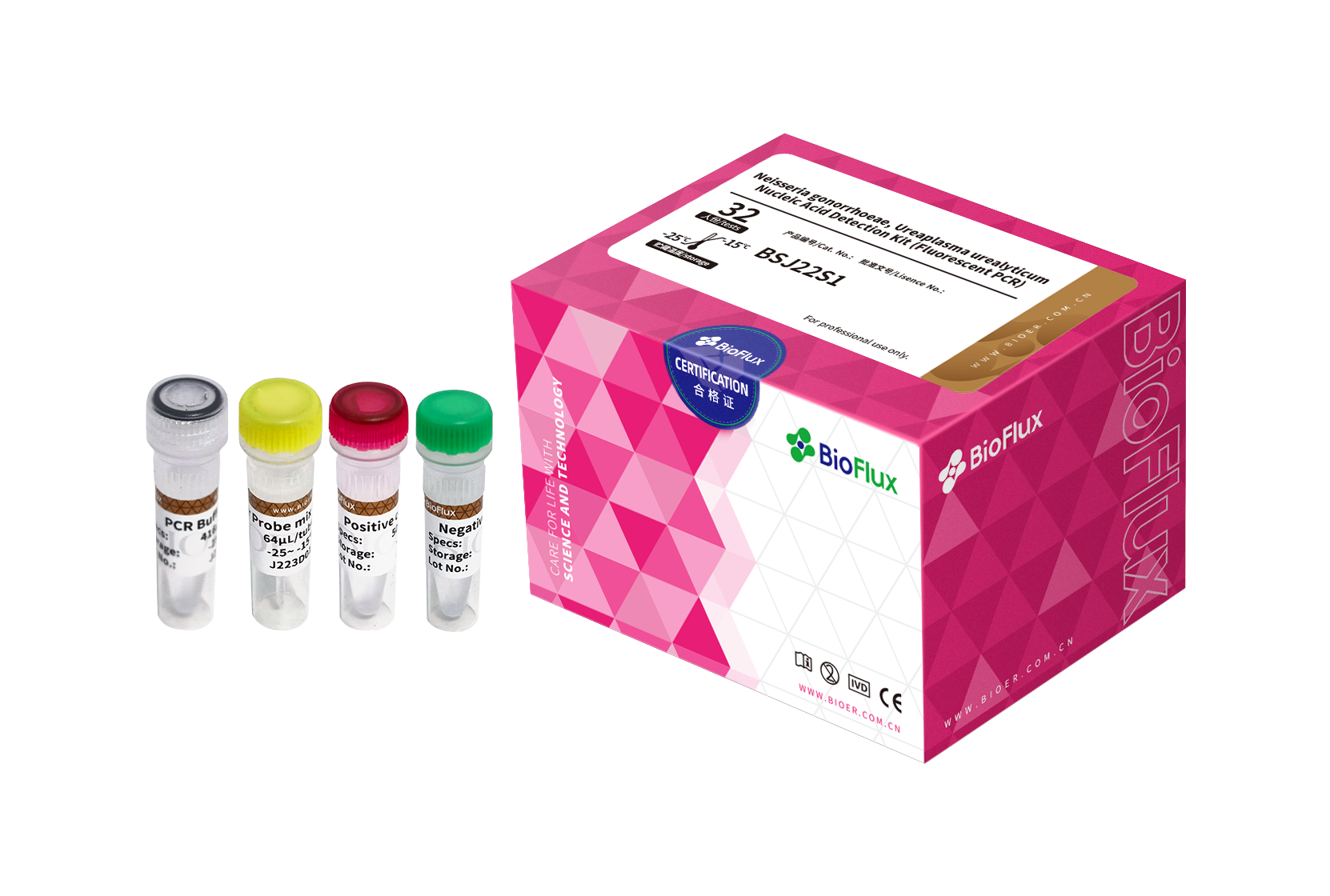
| Cat.No | Product Name | Package |
| BSJ22S1 | Neisseria gonorrhoeae, Ureaplasma urealyticum Nucleic Acid Detection Kit (Fluorescent PCR) | 32T |
Result:The results showed that the coincidence rate of this kit to positive references were 100%, indicating that this kit has excellent performance.
Case 2. The precision references J1 & J2 of ureaplasma urealyticum and J1&J2 of neisseria gonorrhoeae were extracted using Bioer nucleic acid purification reagent and then were tested by this kit. The whole tests were repeated 10 times to verify the precision.