Heavy! Bioer has become one of the two standard setters in the laboratory industry
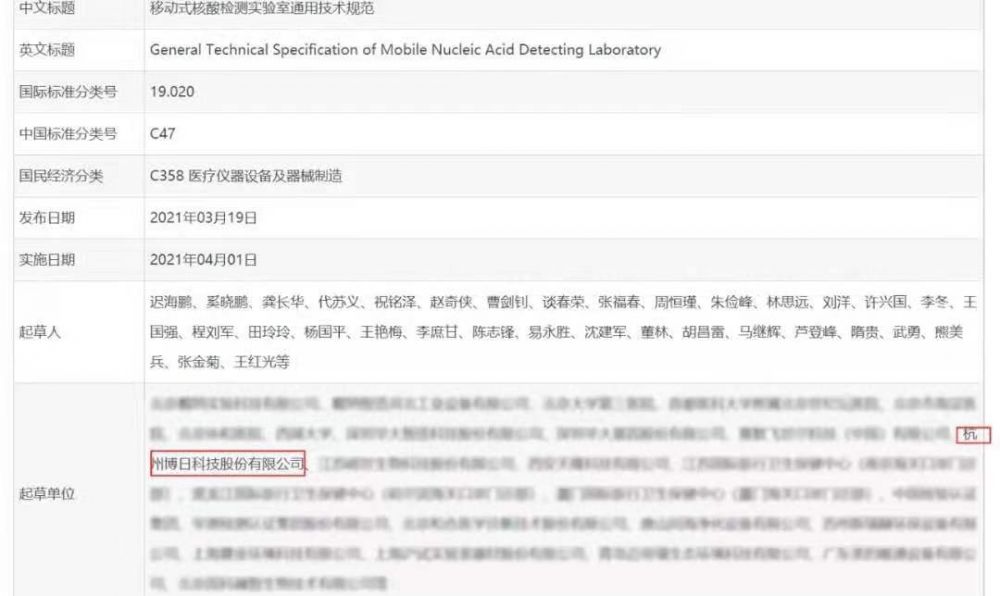
On March 22, 2021, China entry exit inspection and Quarantine Association held a symposium on group standard release, which officially released the world's first two group standards: General technical specifications for shelter nucleic acid testing laboratories (Standard No. T / CIQA 16-2021) and general technical specifications for mobile nucleic acid testing laboratories (Standard No. T / CIQA 17-2021).

Symposium site
The standard is led by the Technical Committee for standardization of laboratory design and construction of China entry exit inspection and Quarantine Association (CIQA / TC 7), including Hangzhou bori Technology Co., Ltd., Beijing Dana Experimental Technology Co., Ltd., the Third Hospital of Peking University, Beijing Union Medical College Hospital, Shenzhen Huada gene Co., Ltd., China Inspection and Certification Group It is jointly drafted and formulated by China testing and Certification Group Co., Ltd. and other units.

Bioer participates in the development of group standards
The release of the standard provides a basis for the design and manufacture of mobile and shelter nucleic acid testing laboratories, fills the gap of such laboratory standards, is of great significance for the standardized production and application of shelter and mobile nucleic acid testing laboratories, is also conducive to the export of China's epidemic prevention equipment and contributes to the control of global epidemic.
First class enterprises set standards
First class enterprises are the standard. As a leader in the PCR industry, Bioer has focused on the PCR industry for more than 25 years and is an "evergreen" player of industry standard setters.
In 2004, the company obtained the product patent of standard PCR Laboratory (Patent No.: 20040109896.9).
In 2010, as an industry benchmarking enterprise, the company participated in the formulation of Chinese pharmaceutical industry standard for PCR instrument.


Participate in the formulation of PCR instrument and Chinese pharmaceutical industry standard
In 2020, Bioer quickly launched a comprehensive epidemic prevention and testing scheme including standard, shelter and mobile nucleic acid testing laboratories, aiming to provide a strong guarantee for the improvement of short-term and long-term quarantine capacity in epidemic prevention areas.
In 2021, the company was invited to become the setter of two industry standards, marking that its professionalism in the field of PCR and molecular diagnosis has been recognized by the industry.

Standard laboratory

Mobile PCR laboratory - integrated solution

Mobile PCR laboratory - vehicle mounted solution
As a leading provider of molecular detection products and services in China, Bioer has always been "innovative" "As the core of enterprise development, Bioer has filled the technical gap in the PCR field for many times, launched a series of differentiated products and promoted the technology upgrading of China's PCR industry. The company will always adhere to the core concept of technological innovation and endless, and actively participate in the formulation of various standards while continuously promoting technological innovation, so as to standardize the market and improve China's competitiveness Contribute to the overall competitiveness of the sub diagnosis industry!
--> Return to list
