Dear Partners of Bioer Technology,
The novel coronavirus epidemic COVID-19 swept the land of China at the beginning of the new year 2020 and disrupted the rhythm of the reunion of families during the Spring Festival. In the face of the sudden epidemic situation, the CPC Central Committee and the State Council of China attached great importance to the scientific deployment, and the prevention and control of the epidemic was formally launched in China.
Precise detection of 2019-nCov nucleic acid is an important basis for clinical diagnosis and rehabilitation of the patients. It is also the judgment basis for the suspected patient or contact to be freed from isolated observation. Precise and reliable detection is essential to solve the epidemic situation. The diagnosis guideline provided by National Health Commission of the People’s Republic China confirmed the novel nucleic acid positive by real time RT-PCR is the standard of diagnosis.

Real Time RT-PCR as the Standard Guideline by National Health Commission of China
Proven Reliable Nucleic Acid Extraction and Detection Result by Bioer Solution
With the epidemic raging, a large number of suspected cases in Wuhan and the whole country need to be confirmed by nucleic acid detection. As a leading enterprise of life science with strong scientific research ability, Bioer team felt our responsilbility and offered to give up the Spring Festival holidays. We efficiently developed Novel Coronavirus (2019-nCoV) Fluorescence RT-PCR Detection Kit. After the validation of clinical samples, the kit was compared with the kit approved by National Medical Products Administration, and the positive and negative identification was totally consistent.
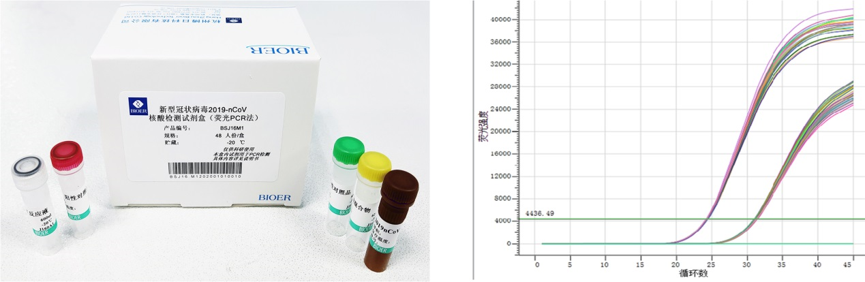
Bioer Novel Coronavirus (2019-nCoV) Fluorescence RT-PCR Detection Kit
In addition to PCR detection, the sample collection of patients is also a very important factor. If the collection process of nucleic acid extraction is not standardized, and the viral load is low, it might result in misdetection of the actual infected case. Bioer’s novel coronavirus nucleic acid extraction and detection solutions are rapidly launched to eliminate these of difficulties. Bioer’ solution provides detailed treatment methods on samples of different types and sizes. Bioer’s nucleic acid purification kits, Nucleic Acid Purification System, 2019-nCov PCR Detection kit and Fluorescent Quantitative Detection System as a total solution ensures the stability of quality and high standard reliability.
Professional Safety Protection, Sufficient Capacity of Production
During the epidemic period, Bioer, as a government designated epidemic prevention and control material production enterprise, started full capacity emergency production and strictly controlled our staff’s safety. Bioer set up novel coronavirus emergency control team, and formulated safety production guidance in immediate time, including at least 3 body temperature measurements per day and plenty supply of medical protection, to ensure our staff’s safety. Due to proper prevention and control, among more than 6700 employees of Bioer technology and our group companies, none of us was infected during the epidemic. Production was promoted in an orderly management, ensuring sufficient supply of key reagents and instruments.

Bioer’s Safety Control and Production Line
Take Social Responsibility, Overcome the Difficulties.
After launching the novel coronavirus nucleic acid extraction and detection solution, Bioer received donation requests from Hubei Provincial Center for Disease Control and prevention.
In January 25, 2020, Bioer’s novel coronavirus detection reagents and emergency supplies donated by Bioer arrived at Wuhan and were immediately used for 2019-nCov virus detection. Up till now, novel coronavirus reagents have been distributed over 2 million yuan.
We contacted the institutions in need of help to issue emergency transport certification, through specialized transportation, aircraft consignment and other means. Despite of difficulties, with duty at our shoulders, Bioer promptly responded to the needs from the front line with efficient mobility, and provided strong support for epidemic prevention and control.
Bioer welcomes partners with professional resources to participate in this global epidemic prevention and control blockade war. If your enterprise has the ability to provide anti-epidemic materials, you can contact Bioer to bring the anti-epidemic frontline to integrate with Bioer’s technology. If you need technical support from Bioer on 2019-nCov nucleic acid extraction and detection, we are ready to provide the best we can. Thank you for your contribution to the protection of human healthy from COVID-19.

